Electrochemistry: Current dependence on scan rate
In this tutorial we use the Rodeostat potentiostat and a screen printed electrode to demonstrate current dependence on scan rate. We know that for freely-diffusible analytes like ascorbate, the anodic peak current depends linearly on the square root of the scan rate. This is described by the Randles-Sevcik equation. The Randles–Sevcik equation describes the effect of scan rate (v) on the peak current (ip) for a cyclic voltammetry experiment.
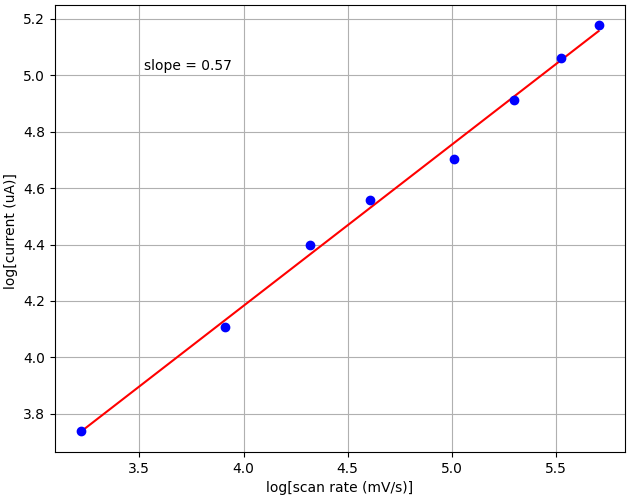
The slope of the fit of log [ip] vs log [scan rate] can suggest whether the process is controlled by diffusion. Diffusion-controlled electrode processes are characterized by value of the slope close to 0.5. However, processes controlled by adsorption are described by a slope close to 1.0 (Suliborska et al., (2019) Proceedings 2019, 11(1), 23).
Materials
- Rodeostat Potentiostat with set of banana cables (included)
- Hyper value screen printed electrode
- Laptop or computer with Rodeostat software installed
- Fresh solution of 10mM ascorbate in 0.1M KCl
Cyclic voltammetry parameters
- Min: -100 mV
- Max: 1000 mV
- Scan rate: 25, 50, 75, 100, 150, 200, 250 and 300 mV/sec
- Sample rate: 100 Hz (samples/sec)
- Cycles: 1
Methods
- Connect a screen printed electrode to the Rodeostat potentiostat using banana cables as shown in the image below
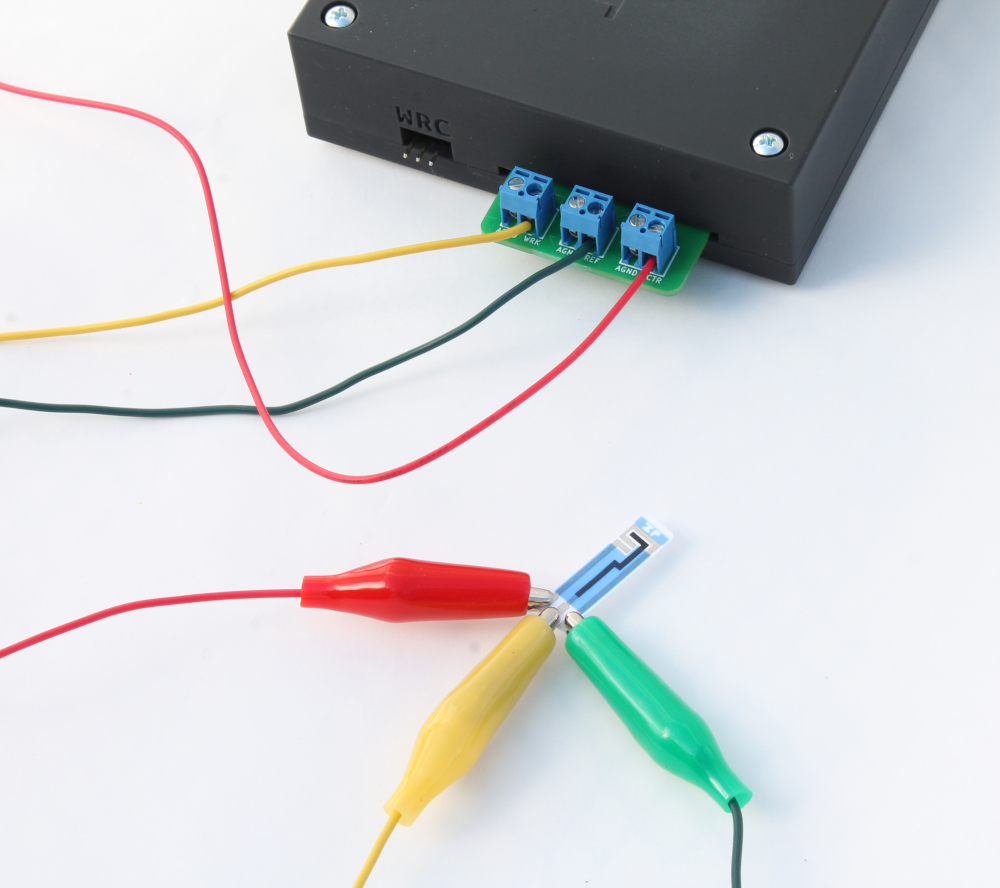
- Pipette 4-5 drops of fresh ascorbate solution onto the working area of the SPE and run a CV using the parameters above and appropriate scan rate e.g. start at 25 mV/sec
- Pipette fresh ascorbate solution between each new scan rate experiment ending at 300 mV/sec
Results
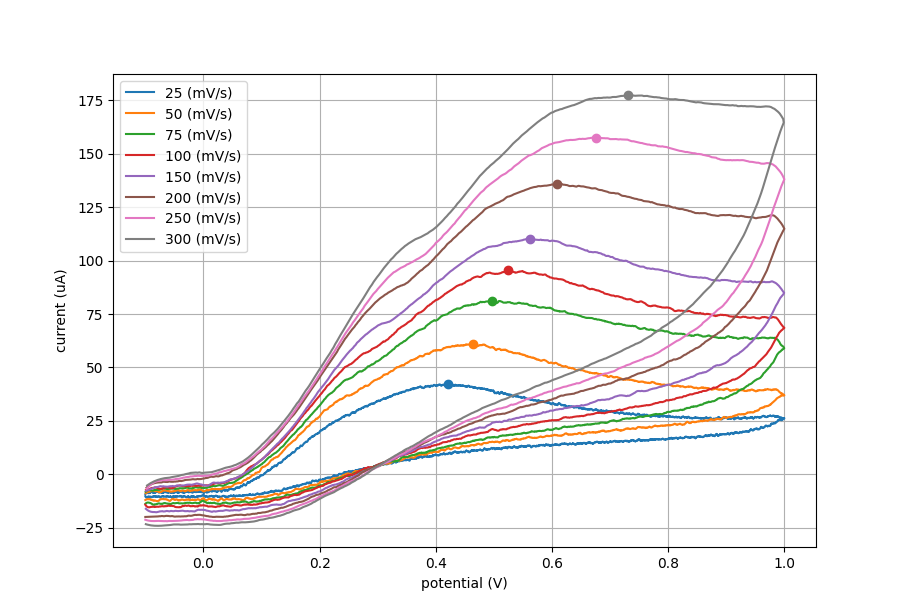
We ran 3 separate trials for this experiment. The same screen printed electrode was used throughout all trials, with similar results. Data from one of these trials is shown in the figures below.
The plot below shows cyclic voltammetry data with increasing scan rate. Using plotting software we can find the peak current (ip) for each CV. This peak current was used in the data for the next two plots.
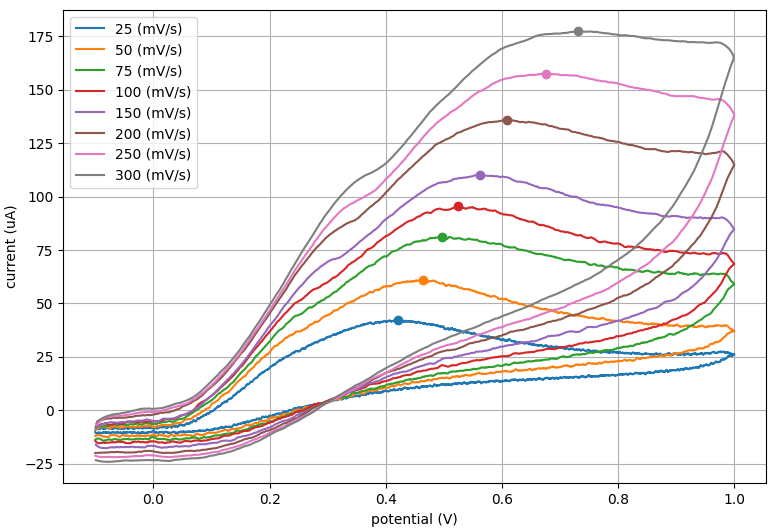
We next examined linearity by plotting peak current versus the square root of the scan rate. You can see from the plot below that there is a clear linear fit between current and square root of scan rate (r2 = 0.993).
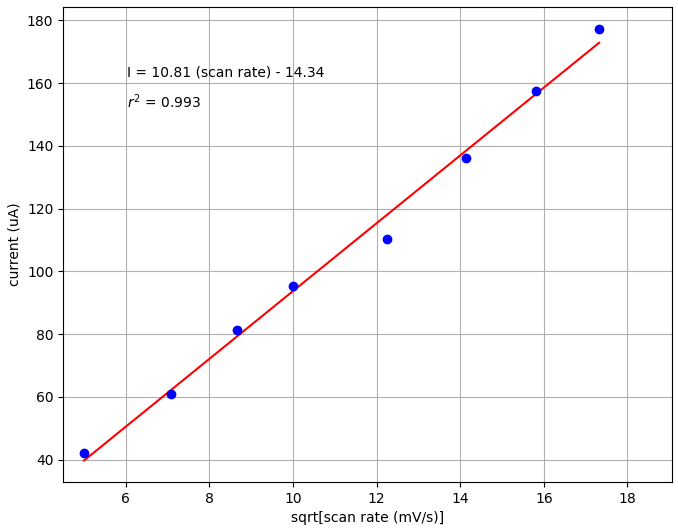
Finally, we wanted to find the slope of the linear dependence between log of peak current and log of scan rate. This can tell us whether a reaction is controlled by diffusion processes or adsorption. The slope is 0.57 in our tests, which is close to the theoretical slope of 0.5 for diffusion processes.
Summary
In this tutorial we used low-cost tools to demonstrate several introductory electrochemistry concepts: Cyclic Voltammetry, Scan Rate, Diffusion and Current Dependence on Scan Rate. The total cost of equipment is $240 for the Rodeostat potentiostat and only $2 for the screen printed electrode used in the tests.
Additional Resources









Comments ()