Measuring blue food dye in sports drinks

Goal: Use the Open Colorimeter to experimentally calculate the molar extinction coefficient of FD&C Blue #1
Beer’s Law states that absorbance of a sample (Abs) depends on the molar concentration (c), light path length in centimeters (l), and molar extinction coefficient (ε) for the dissolved substance at the specified wavelength (λ) as follows:
Molar extinction coefficient (also known as molar absorptivity or attenuation coefficient) is a measure of how strongly a substance absorbs light at a particular wavelength, and is usually represented by the unit M-1 cm-1 or L mol-1 cm-1.
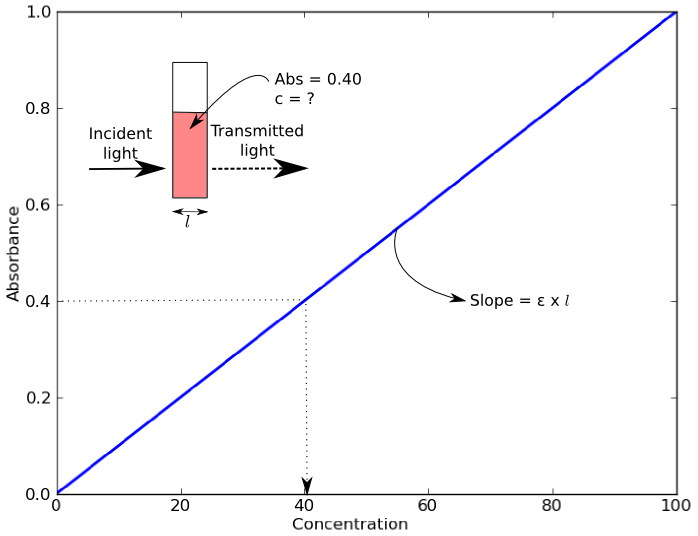
An example of a Beer’s Law plot (concentration versus absorbance) is shown below. The slope of the graph (absorbance over concentration) equals the molar extinction coefficient, εl. As most cuvettes including the ones used in the Open Colorimeter have a 1cm pathlength,( l = 1cm) the slope = molar extinction coefficient.
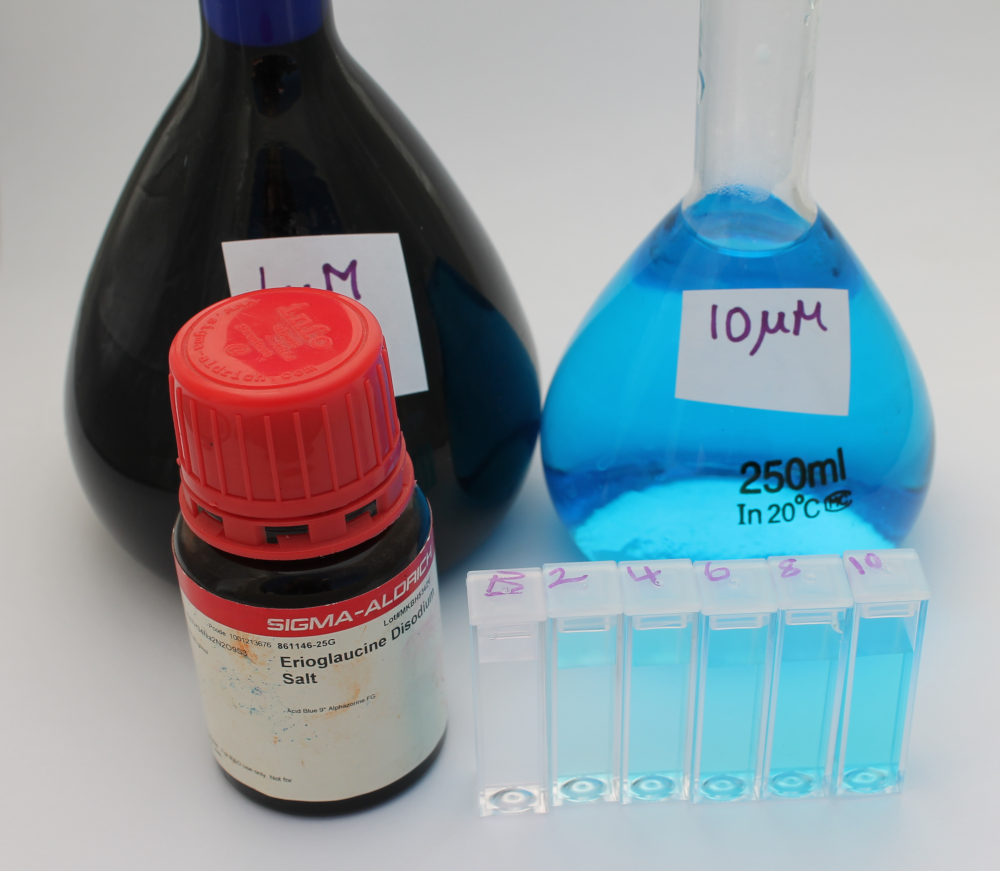
Blue food dye, FD&C Blue 1, is also commonly known as Brilliant Blue or Erioglaucine. It has a molar extinction coefficient at 625nm of 80,000 M-1 cm-1. As we already had Erioglaucine in powdered form we chose to use this to experimentally determine the molar extinction coefficient using the Open Colorimeter and compare this to the reported value of 80,000 M-1 cm-1.
Materials we used
- Erioglaucine Disodium Salt, Sigma Part # 861146-25G (MW: 792.85)
- Open Colorimeter with 632nm red LED
- BrandTech disposable PS macro cuvettes
- Powerade Mountain Berry Blast and Gatorade Zero Cool Blue
- Distilled water (any grocery store)
- Glassware and pipettes
- Measuring scale
Method
- Weigh out 0.396 g of erioglaucine and place in 500 mL volumetric flask. Fill to line with distilled water and label 1mM stock
- Transfer 2.5 mL of the 1mM stock into a 250 mL volumetric flask. Fill to line with distilled water and label 10 µM working stock
- From the 10 µM working stock, prepare 2 µM, 4 µM, 6 µM and 8 µM solutions
- Blank the Open Colorimeter with a distilled water cuvette and measure absorbance of all samples using the red led (632nm)
- Plot data and find the slope with plotting tool of your choice. We are using Matplotlib
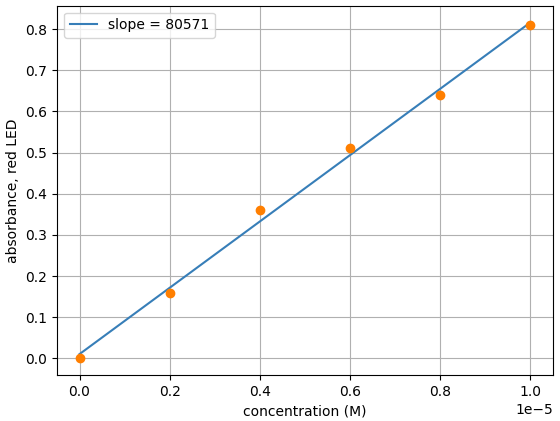
From the slope, we can see the measured molar extinction coefficient for erioglaucine with the Open Colorimeter is 80,571 M-1 cm-1. This is a difference of just 0.7% from the reported value of 80,000 M-1 cm-1.
Experiment 2: Determine the concentration of FD&C Blue 1 with the Open Colorimeter
Goal: Using Beer's Law and the measured molar extinction coefficient (ε), we can use the following equation to determine concentration of Blue 1 in a solution (e.g. sports drink) from absorbance measured at 632nm:

Results
A sample of each drink was poured into a cuvette and absorbance measured at 632nm. Neither sample needed dilution.
- Mountain Berry Blast: Abs = 0.46-0.47; Concentration = 5.7-5.8 µM
- Cool Blue: Abs = 0.49-0.51; Concentration = 6.1-6.3 µM
Using the molecular weight of Blue # 1 (792.85) we can convert this to mg/L:
- 5.75 µM Mountain Berry Blast = 4.56 mg/L (or 3.8 mg per 828 mL bottle)
- 6.2 µM Cool Blue = 4.92 mg/L (or 4.1 mg per 828 mL bottle)


Comments ()