Introduction: Absorbance & Fluorescence Measurements

The Open Colorimeter instruments can be used to measure absorbance, fluorescence and light-scattering as described in this page.
1) Absorbance Measurements
Colorimeters are used to quantify the concentration of an analyte in a sample based on the amount of light of a specific wavelength that is absorbed by the test sample. Absorbance is related to concentration as described by the Beer-Lambert Law. The greater the concentration of a sample, the greater the absorbance value. A light sensor measures the amount of light transmitted through a the sample and calculates absorbance. Comparison of the absorbance measurements to a calibration curve provides the final concentration in the sample. Absorbance (A) of the sample is determined by comparing the intensity of incident light (I0) to the intensity of light after it has passed through the sample (I) using the following equation:
$$ A = -\mathrm{log}_{10}(I/I_0)$$
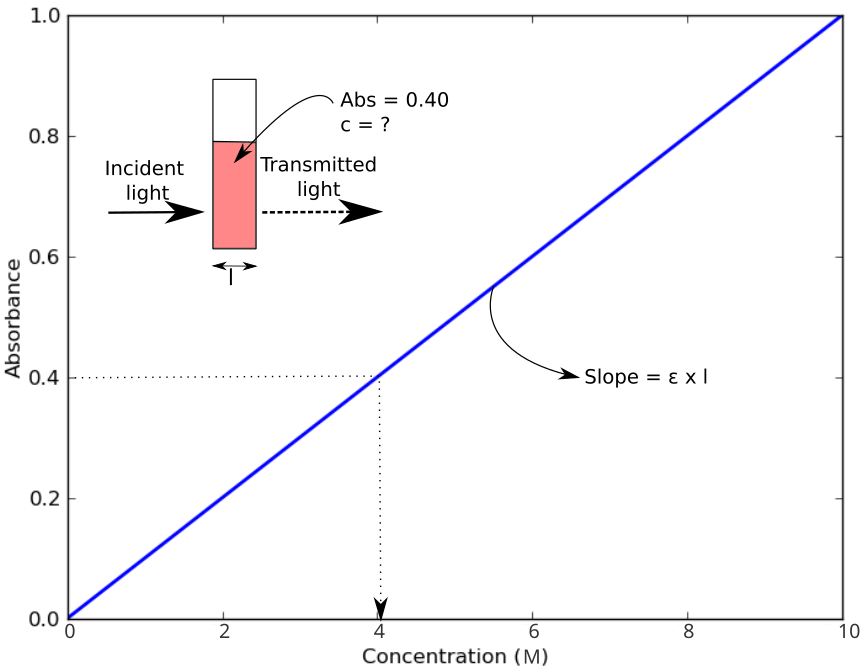
Absorbance measurements are typically coupled with a well-characterized colorimetric assay for a specific analyte. There are often several options of colorimetric assays for each analyte, depending on the measurement range the user needs. More information on this page:
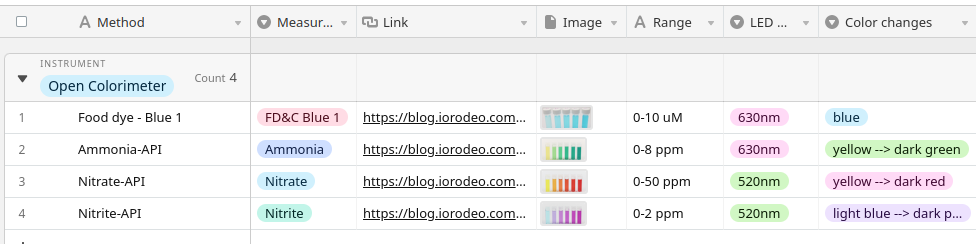
In addition to colorimetric assays, absorbance can be calculated without the use of a color change reaction.
For example absorbance of UV light by proteins and nucleic acids can be easily used for quantification. These are described in more detail in the following posts.


2) Fluorescence Measurements
(in progress)

Open Colorimeter Product Guide
Documentation site for the Open Colorimeter, Multi-Channel Colorimeter, UV Open Colorimeter & Open Colorimeter Plus
Product Guide Home Page