Bismuth modified carbon paste electrode for metal measurements
In the experiments described in this blog post, we made a bismuth modified carbon paste electrode for detecting heavy metals, lead and zinc, using square wave anodic stripping voltammetry and the Rodeostat potentiostat. This is a continuation of our experiments using electrochemistry to measure lead and other heavy metal pollutants in water. You can check out these previous blog posts for more background information:
Materials
- Rodeostat potentiostat
- Graphite powder 20 µm, Sigma, part # 282863-25G
- Bismuth oxide, Sigma, part # 202827-10G
- Mineral oil, Home Science Tools, part # CH-MINROIL
- 1,000 ppm Lead standard - Sigma, part # 16595-250ML
- 1,000 ppm Zinc standard - Sigma, part # 18827-250ML
- Platinum wire counter electrode - CH Instruments, part # CHI115
- Ag/AgCl2 reference electrode - CH Instruments, part # CHI111
- 0.1M Sodium acetate buffer, pH 4.5 - see Square wave anodic stripping voltammetry
- DIY carbon paste electrode holder - see Making a carbon paste electrode
- Electrochemical cell - see Making a custom electrochemical cell
- Ceramic mortar and pestle
- Stir plate & stir bar
- Micropipettes
Preparing a bismuth modified carbon paste electrode
The methods for making an unmodified carbon paste electrode have been previously described in the blog post Making a carbon paste electrode. In this experiment, the only difference is that the mixture was modified with 10% bismuth by mixing 0.1g of bismuth oxide in with 1g of the 70/30 carbon/mineral oil mix. After thoroughly mixing the bismuth/carbon paste in a mortar and pestle, the mix was packed into a carbon paste electrode holder.
Experimental set-up
The bismuth-modified carbon paste electrode was placed into an electrochemical cell along with the Ag/AgCl2 reference electrode and the platiunum wire counter electrode. The beaker was filled with 50 mL of sodium acetate buffer with Zn and Pb added at the concentration being tested. The beaker was placed on a stir-plate with moderate stirring and connected to the Rodeostat. See the blog post Making a custom electrochemical cell for more details on connecting the Rodeostat.
Running the Rodeostat Squarewave anodic stripping voltammetry program
- To run the experiment, first launch the serial-bridge software as described in Serialport-bridge Software
- Once this is running, launch the Rodeostat software by going to the web app here: http://stuff.iorodeo.com/apps/rodeostat/
- The web app has a main menu on the left-hand side (triple horizontal bars). Click on the main menu and you will see a drop-down menu with 3 options as shown below
4. Select "Device Connection" from the drop-down list. Follow the steps to connect the device as described in the blog post Rodeostat Web App Software. Below is an example of a connected device:
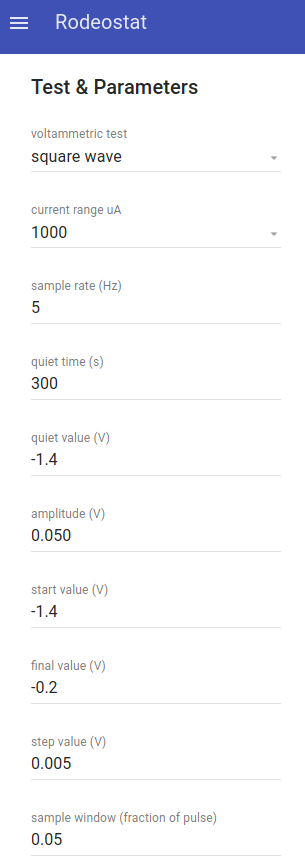
- Next, go back to the main menu and select "Test & Parameters"
- Under the first option for "voltammetric test" select Squarewave from the drop-down list
- Enter the remaining Squarewave parameters. For experiments described in this blog post, the following parameters were used (current range and preconcentration time did vary)
- Next, go back to the main menu and select "Data Aquisition". Click Run Test to run the experiment. Below is an example result. The potential vs time and current vs time are not displayed in this example.
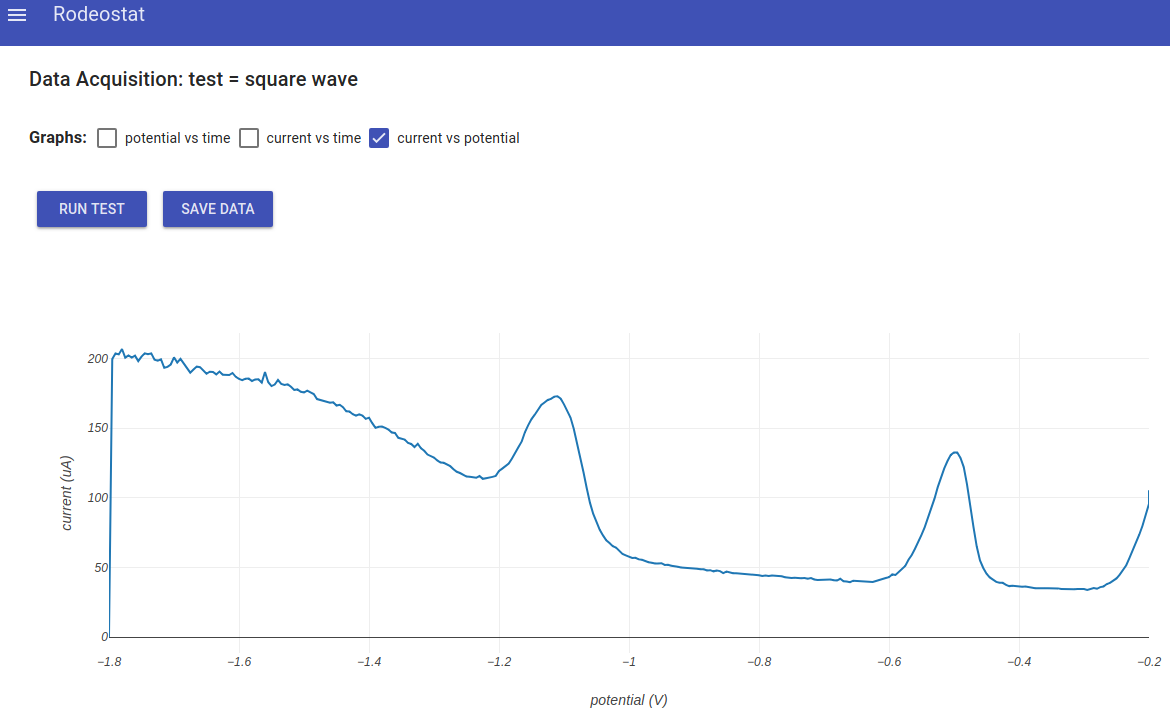
Results
1. Lead detection
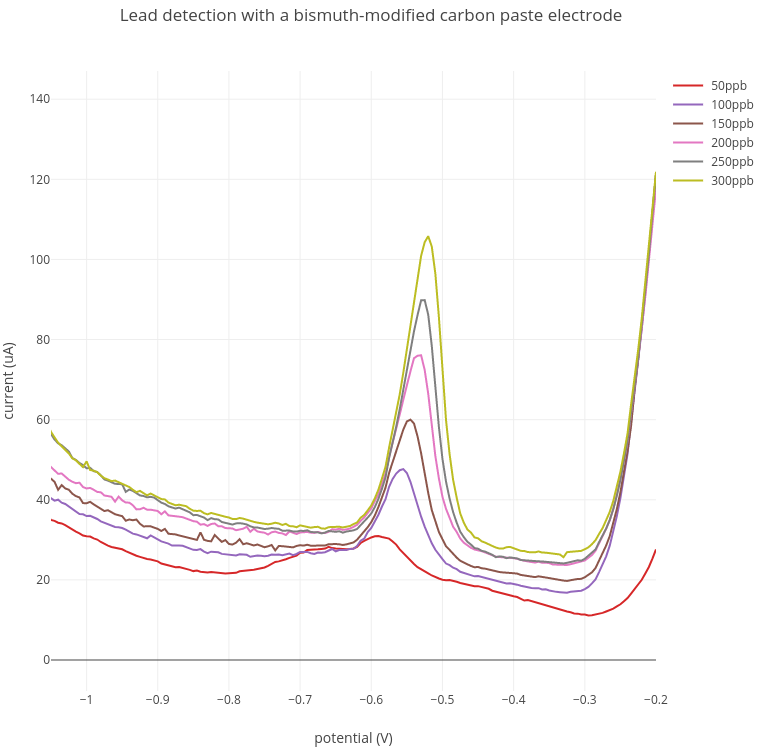
In these experiments, increasing amounts of lead from 50 - 300 ppb were added to the acetate buffer. A lead peak at between -0.52V and -0.57V was detected at around 100 ppb which increased with increasing lead concentration.
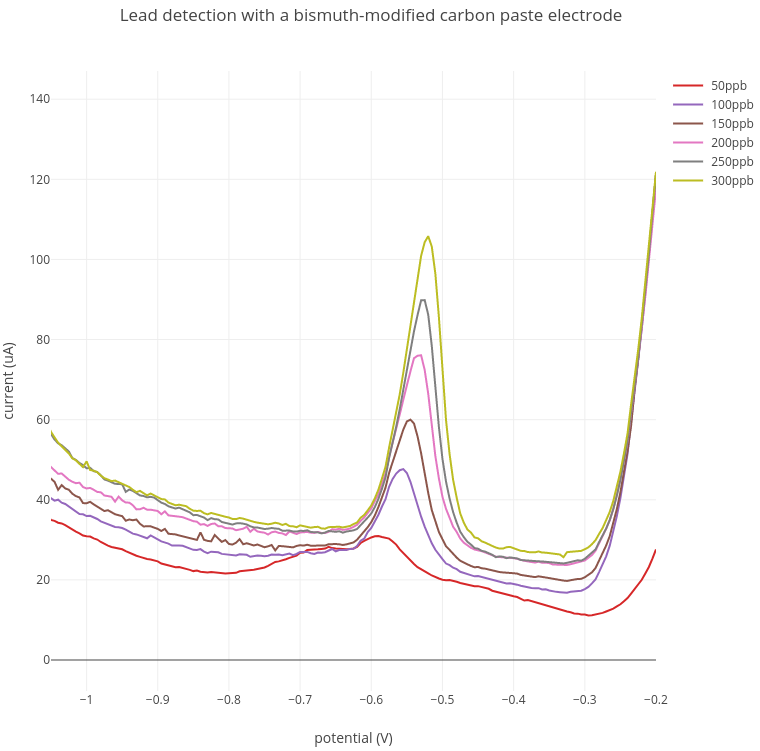
Current range: 100 uA; Preconcentration: 3 min at -1.4V; Stripping: -1.4V to -0.2V.
2. Zinc detection
In this experiment, 200 ppb of lead and 200 ppb of zinc were added to 50mL of sodium acetate buffer. In the graph below, two replicate experiments are shown of the same 200 ppb lead and zinc solution. In this experiment a zinc peak was detected at around -1.15V. A lead peak is detected at around -0.53V as before.
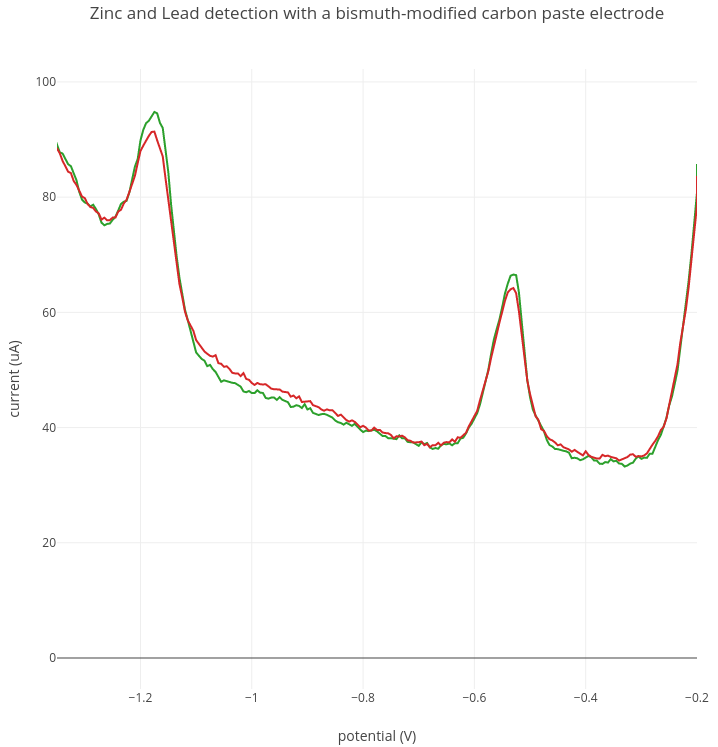
Current range: 1000 uA; Preconcentration: 5 min at -1.4V; Stripping: -1.4V to -0.2V.
Next steps
The goal of this experiment was to determine whether we could detect heavy metals using a homemade bismuth modified carbon paste electrode and the Rodeostat squarewave anodic stripping voltammetry program. From these preliminary experiments we can detect both metals tested - zinc and lead. The zinc peak was detected at around -1.15V, and lead at around -0.54V. The next steps will be to i) Optimize the experimental conditions for metal detection (e.g. bismuth concentration, type of bismuth, pre-concentration time/voltage, buffer) and ii) Determine the range of metals we can detect with this method e.g. the lowest concentration we can detect.


Comments ()